|
Chemistry Class -
XII 2000 (CBSE)
You are on Set 1 questions 21 to 34
Q.21 Among ionic species,
Sc3+, Ce2+ and Ti4+, which ones will be
occurred in aqueous solution? (Atomic number : Sc = 21, Ce = 58, Eu = 63)
(Marks 2)
Q.22 Explain the following processes with a
suitable example in each case :
(i) Chain - growth
polymerisation
(ii) Step - growth polymerisation (Marks 2)
Q.23 Given an example of each, differentiate
between multi-molecular and macromolecular colloides. (Marks
2)
Q.24 What are polysaccharides? Name one of
the. How is it important for us. (Marks 2)
Q.25 What do you understand by the statement
: "ATP molecules are the currency of energy metabolism in a cell."
(Mark 2)
Q.26 Name the broad spectrum antibiotic
and state two diseases for which it is prescribed. (Mark 2)
Q.27 The ionisation energy of hydrogen atom
in ground state is 1312 kJ mol-1. Calculate the wavelength of
radiation emitted when the electron in this atom makes a transition from n
= 2 state to n = 1.
(Planks constant, h = 6.63 x 10-34 Js,
velocity of light = 3 x 1010 cm s-1 and Avogadro
number, NA = 6.02 x 1023 mol-1) (Marks
3)
Q.28 On dissolving 3.24g of sulphur in 4.0g
of benzene boiling point of solution was higher than that of benzene by
0.81 K. Kb value for benzene is 2.53 K kg mol-1.
What is the molecular formula of sulphur ? (Atomic mass of sulphur = 32 g
mol-1) (Marks 3)
Q.29 Calculate the cell emf and 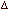 G for the cell reaction
at 25oC for the cell G for the cell reaction
at 25oC for the cell
Zn (s) | Zn2 + (0.0004 M)
|| Cd2+ (0.2 M) | Cd (s)
Eo values at
25o C : Zn2+ / Zn = - 0.763 V ; Cd2+/ Cd = -0.403 V;
F =
96500 C mol-1; R = 8.314 JK -1. (Marks
3)
Q.30 Describe how important functions each
of the elements P, B and S in green plants and also write the deficiency
symptoms of any two of them.
(Marks 3)
Q.31 Describe the following
:
(i) Optical isomerism.
(ii) Magnetic behaviour of
[Ni(CN)4]2- ion. (At. No. of Ni =28)
(iii)
Preparation of tetrabutyl tin (Marks 3)
Q.32 (i) What is nuclear fission reaction
(ii) Describe the basic principle of a nuclear reactor? (Marks 3,
2)
Q.33 (a) Write chemical equation and
reaction conditions for the conversion of :
(i) Propane to
1-bromo-propane
(ii) Chlorobenzene to phenol
(iii) 2-propanol to
iodoform
(b) How is glycerol obtained commercially, state its two uses.
(Mark 3)
Q.34 (a) Account for the following
observation :
(i) In several chemical properties lithium resembles
magnesium.
(ii) +1 oxidation state of thallium (atomic no 81) is more
stable than its +3 oxidation state.
(iii) Boron chloride exists as a
monomer while in the same group anhydrous aluminium chloride exists as
adimer.
(b) Complete and balance the following chemical equation
:
(i) NH3 + NaOCl ---->
(ii) XeF4 +
SbF5 (Mark 3, 2)
|
|
