|
Chemistry Class -
XII 2000 (CBSE)
You are on Set 1 questions 1 to 20
Q.1
What is the physical significance of
the lines in the following depiction of atomic orbitals? (Mark
1)
Q.2 An ionic compound AB2
possesses CaF2 type crystal structure. Write the co-ordination
numbers of A2+ and B- ions in the crystals of AB2.
(AB2+ = 8, B- = 4 ) (Mark 1)
Q.3 How many state of a thermodynamic system
be defined? (Mark 1)
Q.4 State Kohlrausch's law for electric
conductance of an electrolyte at infinite dilution. (Mark 1)
Q.5 For the reaction 3N2 (g) +
H2 (g) ----> 2NH3 (g), how are the rate of
reaction expression -d[H2]/ dt and d[NH3]/dt
interrelated? (Mark 1)
Q.6 Mention the use of fromalin in
industry. (Mark 1)
Q.7 For an amine RNH2
write the expression for Kb to indicate its base strength.
(Marks 1)
Q.8 What is responsible for the blue colour
of a solution of an alkali metal in liquid ammonia? (Mark 1)
Q.9 Why do colloidal solution exhibit
Tyndall effect? (Mark 1)
Q.10 Write any one characteristics feature
of enzymatic catalysts. (Mark 1)
Q.11 Account for the following :
(i)
Silicon is an insulator but silicon doped with phosphorous acts as a
semiconductor.
(ii) Some of the glass objects recovered from ancient
monuments look milky instead of being transparent. (Mark 2)
Q.12 Give an example each of miscible liquid
pairs showing positive and negative deviation from Raoult's law. Give one
reason each for deviation.
(Mark 2)
Q.13 Starting with
thermodynamic relationship 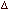 E = q - P E = q - P 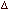 V
and H =E + PV derive the relationship V
and H =E + PV derive the relationship 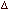 H = qp (Mark
2) H = qp (Mark
2)
Q.14 From
the data given below at 298 K for the reaction :
CH4 (g) +
2O2 (g) ------> CO2 (g) + 2H2O
(l)
Calculate the enthalpy of formation of CH4 (g) at 298
K.
Enthalpy of reaction = - 890.5 kJ
Enthalpy of formation of
CO2 (g) = - 393.5 kJ mol-1
Enthalpy of formation
of H2O (l) = - 286.0 kJ mol-1 (Mark 2)
Q.15 Give the IUPAC names of :
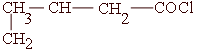 |
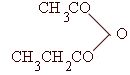 | /
(Mark 2)
Q.16 Give explanation for the
following observation :
(i) An ether would possess a dipole moment even
if the alkyl groups present in it are identical.
(ii) Towards
nucleophilic reagents aldehydes are more reactive tan ketones.
(Mark 2)
Q.17 For the following
conversion reaction write the chemical equations :
(i) Ethyl isocyanide
to ethylamine
(ii) Aniline to N-phenylthanamide(Mark 2)
Q.18 Account for the following :
(i)
Ammonolysis of alkylhalides does not give a corresponding amine in pure
state.
(ii) If -NO2 or -COOH groups is attached to a carbon
of benzene ring, electrophilic substitution becomes difficult. (Marks
2)
Q.19 The sum of first and second ionisation
energies and those of third and fourth ionisation energies of nickel and
platinum are given below :
|
I E +
IE2(MJ mol-1)
| I
E3 + IE4(MJ mol-1) |
Ni |
2.49 |
8.80 |
Pt |
2.66 |
6.70
|
Taking these values into account write :
(i)
The most common oxidation state are for Ni and Pt and its reason.
(ii)
The name of metal (Ni and Pt) which can form compound in +4 oxidation more
easily and why. (Marks 1)
Q.20 (i) Of the ions Ag+,
Co2+ and Ti+, which ones will be coloured in aqueous
solution (Atomic numbers : Ag = 47, Co = 27, Ti = 22)
(ii) If each one
of the above ionic species is in turn place in a magnetic field,
How
will ir respond and why? (Marks 2)
|
