Chemistry Class
- XII 1998 (CBSE)
You are on Set
No. 1 Q.No. 21 to 34
Q21)
Give an example of
each with necessary reaction conditions :
(i) Sandmeyer's
reaction
(ii) Kharasch Effect (Marks 2)
Q22)
What is 'inert pair effect'? Discuss the
oxidation states of Group 13 elements in relation to this
effect. (Marks 2)
Q23)
A co-ordination compound has the formula
CoCl3.4NH3. It does not liberate ammonia but
precipitates chloride ions as silver chloride. Give the IUPAC name of the
complex and write its structure formula. (Marks 2)
Q24)
Write the information asked for in the following
polymers :
(i) Bakelite : material used for preparation
(ii)
Synthetic rubber : monomer unit
(iii) PVC : monomer unit
(iv)
Nylon-66 : materials required for preparation. Marks 1)
Q25)
"The two strands of DNA are not identical but
are complementary." Explain this statement. (Marks 2)
Q26)
Write the structure of adenosine triphosphate
indicating clearly the energy rich bonds. How does this molecule form the
source of energy? (Marks 2)
Q27)
Explain how an electron can be considered to
have a particle as well as nature. A moving electron has 4.9 x
10-25 joules of kinetic energy. Find out its De Broglie
wavelength.
(Given h = 6.6x10-34 Js; m =
9.1x10-31 kg) (Marks 3)
Q28)
An aqueous solution freezes at 272.4 k, while
pure water freezes at 273.0 K. Determine :
(i) the molality of the
solution,
(ii) boiling point of the solution,
(iii) lowering of
vapour pressure of water at 298K.
[ Given Kf = 1.86 K kg
mol-, Kb = 0.512 K kg mol-1 and vapour
pressure of water at 298 K = 23.756 mm Hg ] (Marks 3)
Q29)
(a) When NH4NO2 (s)
decomposes at 373 K, it forms N2(g) and H2O(g). The
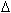 H for the reaction at one
atmosphere pressure and 373k is -223.6 kJ mol-1 of
NH4NO4(s) decomposed. What is the value of H for the reaction at one
atmosphere pressure and 373k is -223.6 kJ mol-1 of
NH4NO4(s) decomposed. What is the value of 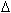 E for the reaction under the same
conditions? E for the reaction under the same
conditions?
[ Given R = 8.31 JK- mol-1 ]
(b)
Account for the fact that 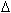 E is
numerically bigger E is
numerically bigger 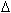 H in the
above case. (Marks 3) H in the
above case. (Marks 3)
Q30)
What is meant by the colloidal state of matter?
Explain the following terms :
(i) Multi-molecular colloids
(ii)
Elector-dialysis (Marks 3)
Q31)
An organic compound A having molecular formula,
C6H6O give a characteristic colour with aqueous
FeCl3 solution. A on treatment with CO2 and NaOH at
400 K under pressure gives B which on acidification gives a compund C. C
reacts with acetyl chloride to give D which is a popular pain-killer.
Deduce the structures of A, B, C and D (Marks 3)
Q32)
(a) Write the balanced equations for the
following reactions
:
450 K
(i) BF3 + LiH .gif)
(ii) NH3 +
NaOCl .gif)
(iii)
SiCl4 + H2O .gif)
(iv) XeF6
+ KF .gif)
(b) State
the reason for the occurrence of "diagonal relationship" in the periodic
table. (Marks 3)
Q33)
(a) Write the general formula for nitroalkanes
and for alkyl nitrites.
(b) How are nitroalkanes prepared
alkanes?
(c) What happens when :
(i) nitroethane is treated with
lithium aluminium hydride,
(ii) nitrobenzene is reduced with hydrogen
using copper oxide as catalyst.
(d) Write two use of nitroalkanes, one
each in the laboratory and in the industry. (Marks 5)
Q34)
(a) (i) What are inner transition elements?
Write their general electronic configuration.
(ii) In what way are the
observed oxidation states of the lanthanide's related to their electronic
configuration?
(b) Describe the general characteristics of the
transition elements with special reference to their tendency to :
(i)
exhibit paramagnetism,
(ii) form complex compounds,
(iii) their
catalytic behaviour. (Marks 5)
|
