Chemistry Class
- XII 1998 (CBSE)
You are on Set
no 1 Q.No. 1 to 20
Time allowed : 3 hours
Maximum
Marks : 70
General Instructions :
(i) All questions are
compulsory.
(ii) Marks for each question are indicated against
it.
(iii) Question number 1 to 10 are very short-answer questions each
of 1 mark. Answer them in about one sentence each.
(iv) Question number
11 to 26 are short-answer questions of 2 marks. Answer them in not more
than 30 words each.
(v) Question number 27 to 32 are short-answer
questions of 3 marks. Answer them in not more than 40 words each.
(vi)
Question number 33 to 34 are long-answer questions of 5 marks each. Answer
them in not more than 70 words each.
(vii) Use Log tables, if
necessary.
(viii) Please write down the serial number of the question
before attempting it.
Q1) Write the MO electronic
configuration of a diatomic molecule having a bond order of three ?
(Marks 1)
Q2) What are
non-stoichiometric compounds ? (Marks 1)
Q3)
Complete the nuclear
reaction expression ? (Marks 1)
20Co59 (d,p)......
Q4)
Write the IUPAC name
of (Marks 1)
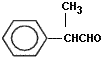
Q5) Why do noble gases form
compounds with fluorine and oxygen only ? (Marks 1)
Q6)
Why is hydrochloric acid
not used to acidify a permanganate solution in volumetric estimations of
Fe2+ or C2O42-?
(Marks 1)
Q7) How many isomers are
there for the complex [Co(NH3)4
Cl2]Cl?
(Marks 1)
Q8)
Why are carbohydrates
generally optically active? (Marks 1)
Q9) Give one important use of
each of the following in pharmacy : (Marks 1)
(i)
Equanil
(ii) Morphine
Q10)
Illustrate the function
of mordent's by one example. (Marks 1)
Q11)
Predict the structure of
MgO crystal and co-ordination number of its cation in which the cation and
anion radii are equal to 65 pm and 140 pm respectively. (Marks
2)
Q12) With the help of
suitable diagrams, illustrate the two types of non-ideal solutions.
(Marks 2)
Q13)
What is meant by average
bond energy? In what way is it different from bond energy of a diatomic
molecule ? Give suitable examples. (Marks 2)
Q14)
Explain Kohlrausch's law
of independent migration of ions. Mention one application of Kohlrausch's
law. (Marks 2)
Q15)
For what concentration
of Ag+ (aq) will the e.m.f. of the given cell be zero at
25oC, if concentration of Cu2+ (aq) is 0.1 M?
(Marks
2)
CU (s) | Cu2+ (0.1 M) || Ag+ (aq) | Ag (s)
[
Given Eo Ag+/Ag = + 0.80 V;
Eo Cu2+/Cu = + 0.34 V
]
Q16)
(a) Show graphically how
the rate of a first order reaction with only one reactant depends upon the
concentration of the reactant.
(b) Give one example of a first order
reaction. (Marks 2)
Q17)
For a reaction, the
energy of activation is zero. What is the value of rate constant at 300 K,
if k = 1.6 x 106 s-1 at 280 K ? [ R = 8.31
JK-1 mol-3 ] (Marks 2)
Q18)
1.0 x 10-6 g
of radioactive iodine is injected into the blood of a patient. How long
will it take for radioactivity to fall to 10% of the initial value?
[
Given t1/2 for 53I133 = 8.05 days ]
(Marks 2)
Q19)
Account for the
following :
(i) Formaldehyde gives Cannizaro's reaction whereas
acetaldehyde does not.
(ii) Carboxylic acids do not give the
characteristic reactions of carbonyl group. (Marks 2)
Q20)
How are the following
conversions carried out?
(i) Ethanoic acid to propanoic acid.
(ii)
Ethanol to propanone.
Write reaction equations and conditions to
support your answer. (Marks 2)
|
