|
Chemistry Class - XII 1999
(CBSE)
You are on questions of Set III
Q.2 In the rock salt structure how many
Na+ ions occupy second-nearest neighbour location of a
Na+ ion? (1 mark)
Q.3 Which type of hybridisation explains the
trigonal bipyramidal shape of SF4? (1 mark)
Q.11 Calculate the uncertainty in the
velocity of a wagon of mass 3000 kg whose position is known to an accuracy
of 10pm. (h = 6.63 x 10-34.Js)
(1 mark)
Q.12 (i) What is the basic building unit of
all silicates?
(ii) How many oxygen atom of this unit are shared with
others in the silicate ion Si3O96-
ion. (1 mark)
Q.23 Draw the structure and write the
hybridisation state of the central atom of each of the following species
:
(i) [Co(NH3)6]3+
(ii)
[NiCl4)]2-) (1 mark)
Q.27 (i) Using the data given below
calculate the value of equilibrium constant for the reaction 3HC =
CH(g) <===> C6H6(g) at 298 K,
issuing ideal gas behaviour.
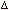 G of HC =
CH(g) = 2.09 x 105 J mol-1 G of HC =
CH(g) = 2.09 x 105 J mol-1
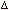 G
of C6H6(g) = 1.24 x 105
J mol-1 G
of C6H6(g) = 1.24 x 105
J mol-1
R = 8.314 J K mol-1
(ii) Based on
your calculated value, comment whether this process can be recommended as
a practical method for making benzene. (3 marks)
Q.29 Give the reasons for the following
features of transition metal chemistry.
(i) The lowest oxide of a
transition metal (say chromium, atomic number 24 ) is basic whereas the
highest oxide is usually acidic.
(ii) Transition metals sometimes
exhibits very low oxidation states such as +1 and 0.
(iii) Zirconium
(atomic number 40) and Hafnium (atomic number 72) occur together in
minerals and they exhibits similar properties. (5 marks)
Q.34 (a) "The first element in a group of
p-block of the periodic table often displays different physical and
chemical properties from the behaviour members of the group". In the light
of this statement give comparative explanations for the following :
(i)
Nature of oxides of boron and aluminium.
(ii) Action of water on
CCl4 and SiCl4.
(iii) Structure's of
N2O5 and P4O10.
(iii)
Maximum numbers of covalency exhibited by oxygen and sulphur.
(b)
Explain the shape of XeF4 on the basis of VSEPR theory. (5
marks)
|
