|
Chemistry Class - XII 1999
(CBSE)
You are on questions of Set II
Q.1 Write the number of unpaired
electron(s) in an atom of an element with the atomic number 21. 9. (1
mark)
Q.3 How many atoms are there in a unit cell
of a metal crystallising in fcc structure?
Q.9 In the formula Fe(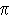 5 -
(C5H5)2 what does the prefix ' 5 -
(C5H5)2 what does the prefix '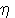 5' denote. 5' denote.
(Mark 1)
Q 12 (i) Draw the structure
of anion (Si6O18)12-.
(ii) How many
oxygen atom of the basic building unit are shared with other units in this
structure? (2 marks)
Q.21 For the first row of transition metals the Eo values are
: (2 marks)
|
V |
Cr |
Mn |
Fe |
Co |
Ni |
Cu |
| Eo(M2+/M) |
-1.18 |
-0.91 |
-1.18 |
-0.44 |
-0.28 |
-0.25 |
+0.34V |
Give suitable explanation for the irregular
trend in these values.
(Mark 1)
Q. 24 Write the state of hybridisation
and the oxidation state of the central atom in each of the following
species : (2 marks)
(i)
cis-[Co(NH3)4Cl2]+
(ii) [Ni(CO)4]
Q. 32 Write an ionic equation for the
conversion of :
(a) Magnate to permanganate.
(b) Permanganate to
manganese.
(c) Chromate to dichromate. (3 marks)
Q.33 Present a comparative account for the
following : (5 marks)
(i) Oxidation state of tin and
lead.
(ii) Structure of pentaoxides of nitrogen and phosphorus
(iii)
Maximum number of covalency exhibited by boron and aluminium.
(iv) Bond
angles of H2O and H2S. (5 marks)
|
