|
Chemistry Class - XII 1996
(CBSE)
You are on Set no 1 Qno. 21 to
34
Q21) What is
the principal of radiocarbon dating? Write one probable source of
error in determining the age of very old pre historic fossils.
(Marks 2)
Q22) What are
elastomers? Give the chemical equation for the preparation of
Buna-S. (Marks 2)
Q23) Explain why
lyophilic sols are relatively more stable then lyophobic sols.
(Marks 2)
Q24) Write and explain
the general structure of triglycerides. (Marks
2)
Q25) What is
glycolysis? Explain. (Marks 2)
Q26) Define the
following: (Marks 2)
(a) Disperse Dyes.
(b)
Antipyretics.
Q27) Calculate the
wavelength of the photon which excites the electron of hydrogen atom
from first shell to fourth shell. Ionisation energy of hydrogen atom
is 1.312 x 10 3 kJ mol-1.
Given:
Planck's constant, h = 6.6 x 1034 kg
m2s-1
Velocity of light, c = 3 x
108 ms-1
Avogadro's number, NA =
6 x 1023 mol-1 (Marks 3)
Q28) Calculate the
equilibrium constant for the following reaction at 298 K and 1
atmosphere pressure :
CO (g) + 3H2(g)
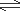 CH4(g) + H2O(l)
CH4(g) + H2O(l)
Given:
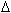 Hf° at 298 K for :
CO(g) = -110.5 kJ mol-1 Hf° at 298 K for :
CO(g) = -110.5 kJ mol-1
CH4(g) = - 74.8kJ
mol-1
H2O (l) = -286.0 kJ mol-1
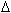 S° for the reaction at 298
K S° for the reaction at 298
K
= -333.3
JK-1 mol-1 Gas constant, R=8.31JK-1
mol-1. (Marks 3)
Q29) The following rate
data were obtained at 300 K for the reaction 2A + B .gif) C + D : C + D :
Experiment
No. |
[A]
mol
L-1 |
[B]
mole
L-1 |
Rate of
formation
of D mol L-1
min-1 |
1
2
3
4 |
0.1
0.3
0.3
0.4 |
0.1
0.2
0.4
0.1 |
6.0 x
10-3
7.2 x 10-2
2.88 x
10-1
2.4 x 10 -2
|
Calculate the rate of
formation of D when [A] = 0.5 mol L-1 and [B] = 0.2 mol
L-1. (Marks 3)
Q30) Write the
resonance structure of phenol (which is similar to that of
chlorobenzene) and explain why phenol is more acidic than ethanol.
Explain whether p-nitrophenol should be more or less acidic than
phenol. (Marks 3)
Q31) Write the
structure of the reagent/organic compound A to F in the following
Sequence of
reactions: (Marks 3)
| |
HNO3
(conc.) |
|
Sn/HCl |
|
CHCl3/
KOH |
|
H2/Pt |
|
| (A) |
.gif) |
(B) |
.gif) |
(C) |
.gif) |
(D) |
.gif) |
(E) |
| |
H2SO4
(conc.) |
|
heat |
 |
heat |
|
heat |
|
| |
|
|
(F) |
 |
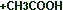 |
|
|
|
Q32) Explain why
transition metals form:
(a) alloys with other transition metals
easily.
(b) compound in different oxidation states.
(Marks 3)
Q33) Explain the
difference between osmotic pressure and vapour pressure of a
solution. (Marks 5)
Q34) Give any three
points of similarities between boron and silicon, and two points of
dissimilarities between boron and aluminum. (Marks
5)
|
